Free Decentralized Clinical Trial Protocol Training Checklists are essential resources for researchers navigating the evolving landscape of clinical research. These checklists offer a structured approach to training staff involved in decentralized trials, ensuring compliance with protocols and ethical guidelines while maintaining data integrity. They’re particularly valuable for teams adopting decentralized methodologies, providing a framework for consistent training across geographically dispersed staff.
Understanding the Need for Free Decentralized Clinical Trial Protocol Training Checklists
Decentralized clinical trials (DCTs) offer numerous advantages, including increased patient access, reduced burden on participants, and accelerated recruitment. However, they also present unique challenges, requiring staff to be proficient in new technologies, data management procedures, and patient interaction methods.  Decentralized Clinical Trial Staff Training Checklist
Decentralized Clinical Trial Staff Training Checklist
Free decentralized clinical trial protocol training checklists provide a standardized approach to equip staff with the necessary skills and knowledge. They help ensure everyone involved understands their roles and responsibilities, minimizing errors and promoting consistent data collection across different locations. These checklists also facilitate regulatory compliance by documenting training activities and demonstrating adherence to good clinical practice (GCP) guidelines.
Key Components of a Free Decentralized Clinical Trial Protocol Training Checklist
A comprehensive checklist should cover various aspects of DCT operations, including:
- Technology Proficiency: Training on the specific platforms and software used in the trial, such as electronic data capture (EDC) systems, telehealth platforms, and wearable sensor technology.
- Data Management: Procedures for collecting, storing, and transmitting data securely in a decentralized environment. This includes training on data privacy and security regulations.
- Patient Engagement: Best practices for communicating with patients remotely, including obtaining informed consent electronically, scheduling virtual visits, and addressing technical issues.
- Regulatory Compliance: Understanding and adhering to relevant regulations and guidelines governing DCTs, such as GCP, HIPAA, and GDPR.
- Ethical Considerations: Addressing ethical implications of DCTs, including patient autonomy, data security, and potential biases.
How to Use a Free Decentralized Clinical Trial Protocol Training Checklist
Implementing these checklists effectively requires a systematic approach:
- Customization: Adapt the checklist to the specific requirements of your trial protocol and the roles of your staff.
- Training Delivery: Utilize a variety of training methods, such as online modules, webinars, and in-person workshops, to cater to different learning styles.
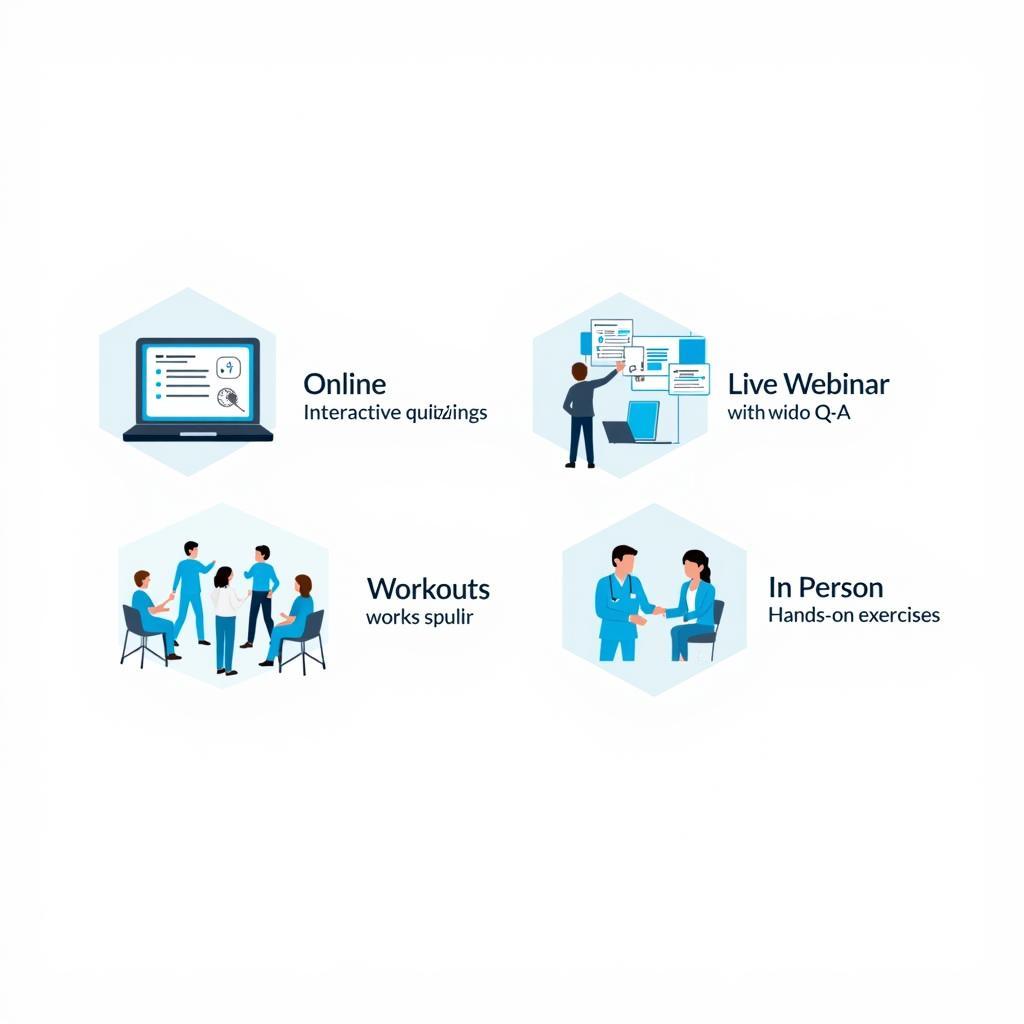 Free Decentralized Clinical Trial Protocol Training Methods
Free Decentralized Clinical Trial Protocol Training Methods - Documentation: Maintain records of all training activities, including attendance, completion dates, and assessment results. This documentation is crucial for demonstrating compliance during audits and inspections.
- Regular Updates: Review and update the checklist periodically to reflect changes in the trial protocol, regulatory requirements, or technology platforms.
Benefits of Using Free Decentralized Clinical Trial Protocol Training Checklists
Utilizing free decentralized clinical trial protocol training checklists offers numerous benefits:
- Improved Data Quality: Standardized training leads to consistent data collection practices, reducing errors and improving the reliability of trial results.
- Enhanced Compliance: Documented training activities demonstrate adherence to regulatory requirements and ethical guidelines.
- Increased Efficiency: Streamlined training processes save time and resources, enabling faster trial startup and execution. free decentralized clinical trials staff training checklist templates
- Improved Patient Experience: Well-trained staff can provide better support and communication to patients participating in decentralized trials.
“A well-structured training program is paramount to the success of any decentralized clinical trial. Free checklists provide a valuable tool for ensuring consistent training across dispersed teams, ultimately leading to better data quality and patient outcomes,” says Dr. Emily Carter, a leading expert in decentralized clinical trials.
Finding Free Decentralized Clinical Trial Protocol Training Checklists
Several resources offer free decentralized clinical trial protocol training checklists:
- Professional Organizations: Many professional organizations involved in clinical research provide free templates and resources for DCT training.
- Open-Access Journals: Some journals publish articles and supplements that include sample checklists and training materials.
- Online Communities: Online forums and communities dedicated to clinical research can be valuable sources for sharing and accessing free resources.
Conclusion
Free decentralized clinical trial protocol training checklists are indispensable tools for ensuring the success of DCTs. By providing a structured approach to training, they contribute to improved data quality, enhanced compliance, and a better overall patient experience. Implementing these checklists effectively is crucial for navigating the complexities of decentralized research and realizing the full potential of this innovative approach to clinical trials. free decentralized clinical trials staff training checklist templates
FAQ
- Where can I find free decentralized clinical trial protocol training checklists?
- How do I customize a checklist for my specific trial?
- What are the key components of a comprehensive checklist?
- How can I effectively deliver training using these checklists?
- What are the benefits of using these checklists?
“These free checklists are not just about ticking boxes; they’re about empowering staff to confidently navigate the complexities of decentralized trials, ensuring ethical conduct and high-quality data,” adds Dr. Michael Davis, a seasoned researcher with extensive experience in DCTs.
When you need support, please contact Phone Number: 0972669017, Email: [email protected] Or visit the address: 142 Tran Nhan Tong, Yen Thanh, Uong Bi, Quang Ninh, Vietnam. We have a 24/7 customer service team.